Something must be done to combat this public health hazard. In 2000, the National Heart Lung, and Blood Institute (NHLBI) began requiring that researchers publicly register their research analysis plan before starting their clinical trials. From a new PLOS paper:
We identified all large NHLBI supported RCTs between 1970 and 2012 evaluating drugs or dietary supplements for the treatment or prevention of cardiovascular disease.
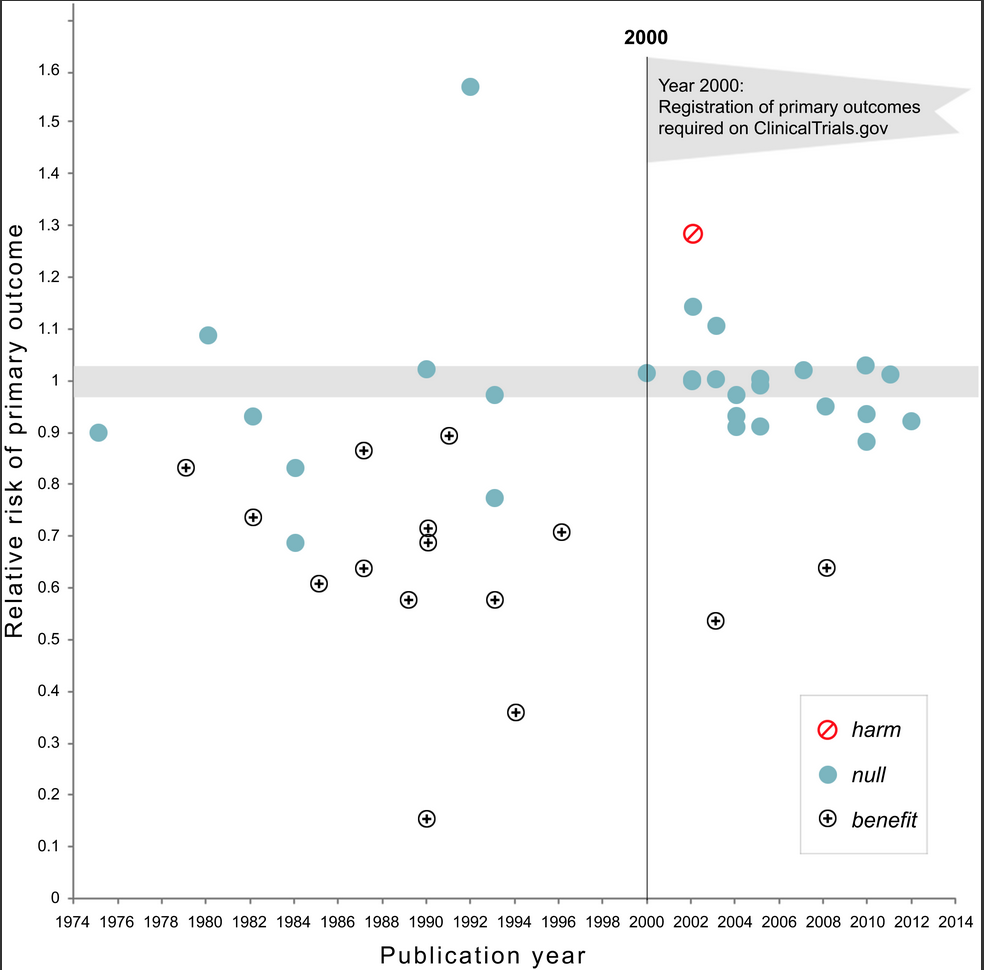
17 of 30 studies (57%) published prior to 2000 showed a significant benefit of intervention on the primary outcome in comparison to only 2 among the 25 (8%) trials published after 2000 (χ2=12.2,df= 1, p=0.0005). There has been no change in the proportion of trials that compared treatment to placebo versus active comparator. Industry co-sponsorship was unrelated to the probability of reporting a significant benefit. Pre-registration in clinical trials.gov was strongly associated with the trend toward null findings.
Hat tip @rlmcelreath.

139 Responses
If pre-registration had been required in 1897 we would have never discovered penicillin.
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @drcjar: Requiring pre-registration of primary outcomes makes the drugs stop working https://t.co/oWnGjjvLPW
Requiring pre-registration of primary outcomes makes the drugs stop working https://t.co/oWnGjjvLPW
Preregistration of clinical trials causes medicines to stop working! https://t.co/xDn2BW8RWm
RT @bill_easterly: Found a lot more significant effects in the years before pre-registry of analysis plans https://t.co/5p4050MGr1 https://…
RT @bill_easterly: Found a lot more significant effects in the years before pre-registry of analysis plans https://t.co/5p4050MGr1 https://…
Pre-registering clinical trials causes medicines to stop working aka p-fishing gone wild https://t.co/V1P4kxhMyV
Pre-registration in clinical https://t.co/ToP3qKjWq8 was strongly associated with the trend toward null findings. https://t.co/PDe8YmL5dI
RT @bill_easterly: Found a lot more significant effects in the years before pre-registry of analysis plans https://t.co/5p4050MGr1 https://…
RT @JustinWolfers: Preregistration of clinical trials causes medicines to stop working!
https://t.co/X8i6uRHnOK @cblatts https://t.co/zEArZ…
This whole Empiric Method thing is bloody dangerous! Once people plan studies in advance, medicines stop working!
https://t.co/XRz8cDxUay
RT @bill_easterly: Found a lot more significant effects in the years before pre-registry of analysis plans https://t.co/5p4050MGr1 https://…
RT @JustinWolfers: Preregistration of clinical trials causes medicines to stop working!
https://t.co/X8i6uRHnOK @cblatts https://t.co/zEArZ…
Preregistration of clinical trials causes medicines to stop working! – Chris Blattman https://t.co/UBiVr5owR7
RT @sjoerdmb: I like explorative stuff, but cant ignore this: Preregistration of clinical trials causes medicines to stop working! https://…
RT @AllenDowney: Looks like preregistration of clinical trials might be fostering better medical science. So that’s good. https://t.co/LU…
RT @economeager: That graph is the best argument for pre-registration of primary outcomes I have ever seen: https://t.co/aAk9UgfhBi
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @j_kalla: Preregistration of clinical trials causes medicines to stop working! From @cblatts https://t.co/Rr47yXIYFB
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
https://t.co/y21p0SmFoF
RT @tukopamoja: “Preregistration of clinical trials causes medicines to stop working!” https://t.co/IoaVER9OLG from @CBlatts https://t.co/s…
RT @bill_easterly: Found a lot more significant effects in the years before pre-registry of analysis plans https://t.co/5p4050MGr1 https://…
RT @neuroconscience: Health crisis! Preregistration of trials causes medicines to stop working! https://t.co/bO7oC9wNUV via @ajshackman htt…
RT @tukopamoja: “Preregistration of clinical trials causes medicines to stop working!” https://t.co/IoaVER9OLG from @CBlatts https://t.co/s…
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
Preregistration of clinical trials causes medicines to stop working! – Chris Blattman https://t.co/0vwH0nO6Vl
RT @johnmyleswhite: Before and after preregistration of studies: https://t.co/y9dltL3sR2
Preregistration of clinical trials causes medicines to stop working! – Chris Blattman https://t.co/eaDFImOMuP
RT @neuroconscience: Health crisis! Preregistration of trials causes medicines to stop working! https://t.co/bO7oC9wNUV via @ajshackman htt…
RT @DrDonaldJTrump: 2/2 we have WEAK medicines and loser pharma has been LYING. And here’s proof https://t.co/KXXv2KBasZ @cblatts we need b…
RT @DrDonaldJTrump: 2/2 we have WEAK medicines and loser pharma has been LYING. And here’s proof https://t.co/KXXv2KBasZ @cblatts we need b…
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @DrDonaldJTrump: 2/2 we have WEAK medicines and loser pharma has been LYING. And here’s proof https://t.co/KXXv2KBasZ @cblatts we need b…
RT @lakens: Preregistration of clinical trials causes medicines to stop working. https://t.co/uSJiafE8y1 (not really, it just stops people …
RT @bill_easterly: Found a lot more significant effects in the years before pre-registry of analysis plans https://t.co/5p4050MGr1 https://…
RT @lakens: Preregistration of clinical trials causes medicines to stop working. https://t.co/uSJiafE8y1 (not really, it just stops people …
RT @lakens: Preregistration of clinical trials causes medicines to stop working. https://t.co/uSJiafE8y1 (not really, it just stops people …
RT @neuroconscience: Health crisis! Preregistration of trials causes medicines to stop working! https://t.co/bO7oC9wNUV via @ajshackman htt…
RT @neuroconscience: Health crisis! Preregistration of trials causes medicines to stop working! https://t.co/bO7oC9wNUV via @ajshackman htt…
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @johnmyleswhite: Before and after preregistration of studies: https://t.co/y9dltL3sR2
RT @neuroconscience: Health crisis! Preregistration of trials causes medicines to stop working! https://t.co/bO7oC9wNUV via @ajshackman htt…
RT @neuroconscience: Health crisis! Preregistration of trials causes medicines to stop working! https://t.co/bO7oC9wNUV via @ajshackman htt…
RT @neuroconscience: Health crisis! Preregistration of trials causes medicines to stop working! https://t.co/bO7oC9wNUV via @ajshackman htt…
RT @tukopamoja: “Preregistration of clinical trials causes medicines to stop working!” https://t.co/IoaVER9OLG from @CBlatts https://t.co/s…
RT @neuroconscience: Health crisis! Preregistration of trials causes medicines to stop working! https://t.co/bO7oC9wNUV via @ajshackman htt…
RT @neuroconscience: Health crisis! Preregistration of trials causes medicines to stop working! https://t.co/bO7oC9wNUV via @ajshackman htt…
RT @neuroconscience: Health crisis! Preregistration of trials causes medicines to stop working! https://t.co/bO7oC9wNUV via @ajshackman htt…
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @neuroconscience: Health crisis! Preregistration of trials causes medicines to stop working! https://t.co/bO7oC9wNUV via @ajshackman htt…
RT @neuroconscience: Health crisis! Preregistration of trials causes medicines to stop working! https://t.co/bO7oC9wNUV via @ajshackman htt…
RT @johnmyleswhite: Before and after preregistration of studies: https://t.co/y9dltL3sR2
Health crisis! Preregistration of trials causes medicines to stop working! https://t.co/bO7oC9wNUV via @ajshackman https://t.co/wLIPAQZXnn
RT @tukopamoja: “Preregistration of clinical trials causes medicines to stop working!” https://t.co/IoaVER9OLG from @CBlatts https://t.co/s…
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
Preregistration of clinical trials causes medicines to stop working. https://t.co/uSJiafE8y1 (not really, it just stops people p-hacking).
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
“Preregistration of clinical trials causes medicines to stop working!” https://t.co/FhXZd9nrZl
RT @j_kalla: Preregistration of clinical trials causes medicines to stop working! From @cblatts https://t.co/Rr47yXIYFB
RT @tukopamoja: “Preregistration of clinical trials causes medicines to stop working!” https://t.co/IoaVER9OLG from @CBlatts https://t.co/s…
RT @j_kalla: Preregistration of clinical trials causes medicines to stop working! From @cblatts https://t.co/Rr47yXIYFB
RT @j_kalla: Preregistration of clinical trials causes medicines to stop working! From @cblatts https://t.co/Rr47yXIYFB
RT @johnmyleswhite: Before and after preregistration of studies: https://t.co/y9dltL3sR2
RT @johnmyleswhite: Before and after preregistration of studies: https://t.co/y9dltL3sR2
RT @j_kalla: Preregistration of clinical trials causes medicines to stop working! From @cblatts https://t.co/Rr47yXIYFB
RT @johnmyleswhite: Before and after preregistration of studies: https://t.co/y9dltL3sR2
RT @johnmyleswhite: Before and after preregistration of studies: https://t.co/y9dltL3sR2
RT @johnmyleswhite: Before and after preregistration of studies: https://t.co/y9dltL3sR2
RT @j_kalla: Preregistration of clinical trials causes medicines to stop working! From @cblatts https://t.co/Rr47yXIYFB
RT @jessefrederik: Preregistration of clinical trials causes medicines to stop working! – Chris Blattman https://t.co/NHZe86J1ct https://t.…
RT @tukopamoja: “Preregistration of clinical trials causes medicines to stop working!” https://t.co/IoaVER9OLG from @CBlatts https://t.co/s…
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @jessefrederik: Preregistration of clinical trials causes medicines to stop working! – Chris Blattman https://t.co/NHZe86J1ct https://t.…
RT @jessefrederik: Preregistration of clinical trials causes medicines to stop working! – Chris Blattman https://t.co/NHZe86J1ct https://t.…
RT @tukopamoja: “Preregistration of clinical trials causes medicines to stop working!” https://t.co/IoaVER9OLG from @CBlatts https://t.co/s…
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
Preregistration of clinical trials causes medicines to stop working! – Chris Blattman https://t.co/NHZe86J1ct https://t.co/vIZPODLbMO
RT @tukopamoja: “Preregistration of clinical trials causes medicines to stop working!” https://t.co/IoaVER9OLG from @CBlatts https://t.co/s…
RT @tukopamoja: “Preregistration of clinical trials causes medicines to stop working!” https://t.co/IoaVER9OLG from @CBlatts https://t.co/s…
For those who don’t click through to the article: note that there is a “harm” outlier in 1991 omitted from the figure reproduced here.
“Preregistration of clinical trials causes medicines to stop working!” https://t.co/IoaVER9OLG from @CBlatts https://t.co/sJMO0MHtmS
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
Pretty clear: Obligation to pre-register clinical trials led to more null results. https://t.co/ELVffniOAW (PLOS One, via @cblatts)
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
Preregistration of clinical trials causes medicines to stop working! https://t.co/H2p6jUiDx1
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY
RT @cblatts: This is what happens when you make medical researchers preregister their clinical trials https://t.co/wdB9RAzUcY